Technical introduction
Crystal purification technology
1) Recrystallization technology for quality improvement
Quality can be improved drastically by recrystallization under strict production and quality control.
(Reference) Impurity levels of our magnesium sulfate before and after recrystallization.
The table can be scrolled horizontally
| Item |
Analysis example (before recrystallization) |
Analysis example (after recrystallization) |
Analysis method |
| Ca |
100ppm |
1.0ppm |
Inductively coupled plasma
atomic emission spectrometry |
| Fe |
0.1ppm |
Below detection
limit(0.1ppm max.) |
〃 |
| Mn |
0.03ppm |
Below detection
limit(0.02ppm max.) |
〃 |
| Na |
2.7ppm |
0.8ppm |
Spectrometry |
| K |
4.4ppm |
0.9ppm |
〃 |
2) Purification technology by HPHT(high pressure /high temperature)
In the state of hydrothermal solution, which is exposed to high pressure and high temperature (HPHT)such as above 100 ℃, reaction progresses quickly in an autoclave ,leading to high degree of crystallization. In case of magnesium hydroxide, HPHT environment creates highly purified crystals with much smoother surface, compared with crystals obtained under normal pressure/temperature conditions.
(Reference) Crystallization of magnesium hydroxide by HPHT
The table can be scrolled horizontally
| Item |
Analysis of crystal obtained under HPHT conditions |
Analysis of crystal obtained under normal pressure /temperature conditions |
Analysis method |
| Purity |
99.7% |
97.3% |
Chelate titration |
| Ca |
6.0ppm |
87ppm |
Inductively coupled plasma
atomic emission spectrometry |
| Fe |
3.8ppm |
12.9ppm |
〃 |
| Mn |
1.8ppm |
3.4ppm |
〃 |
| Na |
41ppm |
89ppm |
Atomic absorption spectrometry |
| K |
21ppm |
61ppm |
〃 |
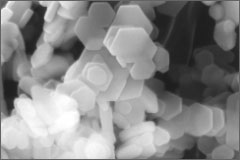
Electron micrograph
of crystals under HPHT conditions
Crystal control technology
1) Crystal form control by habit modification
In case of crystallization of industrial chemicals, most chemical solutions contain impurities. Some impurities work on precipitates to change characteristics of obtained crystal or crystallization behaviors. This phenomenon is called habit modification and those impurities are defined as habit modifiers.
One form of habit modification is the change of crystal form ,which is caused by habit modifiers adhering to specific crystal face growing in a supersaturated solution.
■Example of crystal form change of magnesium sulfate 7-hydrate by habit modification

Electron micrograph
of crystals growing under HPHT conditions

Crystal form of magnesium sulfate
7-hydrate controlled by habitmodification(1)
Crystal form of magnesium sulfate
7-hydrate controlled by habit modification(2)
2)Spherical flocculation technology by reactive crystallization
Reactive crystallization is an operation to generate particles by reaction. In a reaction, a crystal nucleus is generated by producing the state of supersturation and ,at the same time, grow produced crystals by spherical agglomeration. Reactive crystallization is applicable to produce spherical crystals of such chemicals as hardly soluble carbonates and hydroxides.
■Example of spherical crystals of hydroxides obtained by reactive crystallization
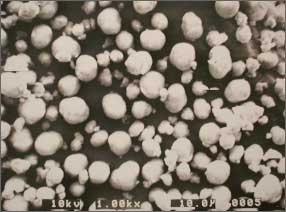
Electron micrograph
of spherically flocculate magnesium hydroxide
by reactive crystallization
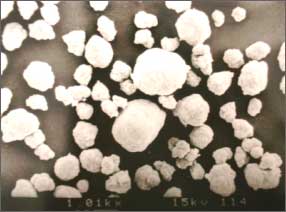
Electron micrograph
of spherically flocculate hydroxide
by reactive crystallization
3) Crystal form control technology by HPHT
Crystal form is controlled in the state of hydrothermal solution under HPHT conditions.
High pressure lowers solubility of compound to crystallize. In the case of water soluble system, it is possible to obtain single crystals of such form that cannot be created under normal pressure/ temperature conditions as crystallization takes place under HPHT conditions. Crystallization is sometimes accompanied by reaction.
■Example of inorganic compound crystals produced under HPHT conditions

(Needle crystals)

(Rhombohedral crystals)

(Hexagonal plate crystals)
Generation of crystal nucleus and crystal growth
Crystallization is a phenomenon realized by both of crystal nucleus generation and crystal growth.
Crystals are not generated immediately after supersaturated solution reaches saturation point by cooling. Crystal nucleus start to appear only after supersaturation becomes somewhat high. Supersolubility is a point for crystal nucleus generation to start. The area of solubility curve between solubility and supersolubility is called metastable domain. After the start of crystal nucleus generation, crystals continue to grow as long as solubility curve stays in this domain. However, the diameter of crystals becomes smaller when supersaturation is too high even if solubility curve is in a metastable domain. Bigger crystals can be obtained when supersaturation is low. Supersolubility changes, depending on the kind of solutions, volume of crystal nucleus, degree of agitation and so on. Industrial crystallization requires adequate crystallization conditions which enable reproduction.
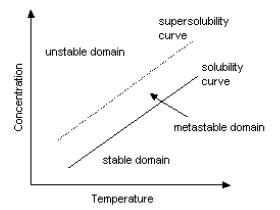
[ Solubility curve
and supersolubility curve ]
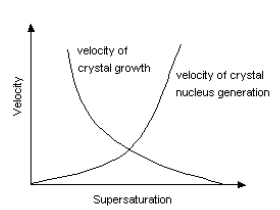
[ The relation between velocity
of crystal nucleus generation and velocity of crystal growth ]
Approach applying crystallization technology
Umai Chemical has been engaged in research and development of various compounds with properties or shapes which are different from conventional compounds by taking advantage of HPHT technology and reaction crystallization technology. The following are examples.
1) Complex hydroxide
By mixing a certain compound into magnesium hydroxide with extremely small particle size to cause hydrothermal reaction, it is possible to produce a special complex magnesium hydroxide which starts to decompose at the temperature 50℃ lower than magnesium hydroxide. It has more removed water content than magnesium hydroxide by about 6%.
his complex hydroxide was developed as a flame retardant for polyethylene. Its properties go well with the ignition point and the flash point of polyethylene(350℃) and it has better incombustibility, compared with the conventional flame retardant of magnesium hydroxide

Electron micrograph
of complex hydroxide (2000 times)
■Examples of differential thermal analysis(DTA)
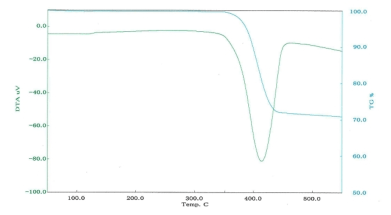
DTA of magnesium hydroxide
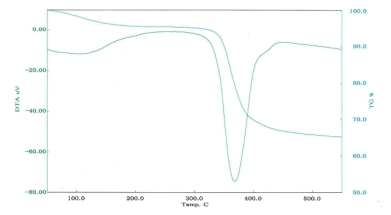
DTA of complex magnesium hydroxide
The table can be scrolled horizontally
| Item |
Analysis of complex hydroxide |
Analysis of magnesium hydroxide |
| Water content |
36.8% |
30.8% |
| Decomposition temperature |
367℃ |
420℃ |
2) Decomposition temperature
Umai Chemical developed spherical magnesium hydroxide by reaction crystallization technology which is characterized by causing reaction in state of supersaturation to produce crystal nucleus while growing so produced crystal nucleus by spherical flocculation.
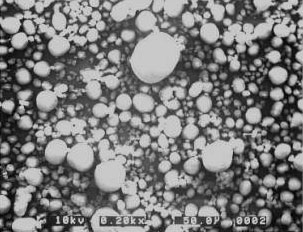
Electron micrograph
of spherical magnesium hydroxide (200times)
The table can be scrolled horizontally
| Item |
Analysis of spherical magnesium hydroxide |
Analysis method |
| Particle size |
~100μm |
Electron microscope |
| High purity magnesium hydroxide |
98.7% |
Chelatometric titration |
| Ignition loss |
30.1% |
500℃ 3hours |
| Ca |
0.01% |
Inductively coupled plasma-atomic emission spectrometry |
| Na |
0.05% |
〃 |
| K |
0.01%以下 |
〃 |
| Fe |
5ppm以下 |
〃 |
Approach to Apply Powder Processing Technology
We are working on the development of powders with controlled particle size and shape by applying powder processing technology, which is one of advanced functional technologies. The followings are the examples of our results.
1) Fine Anhydrous Magnesium Sulfate SSN-00
Magnesium sulfate anhydrate is pulverized finely with a special crusher. An average particle size of approximately 6 μm is realized.

Electron micrograph of SSN-00 (2000 times)
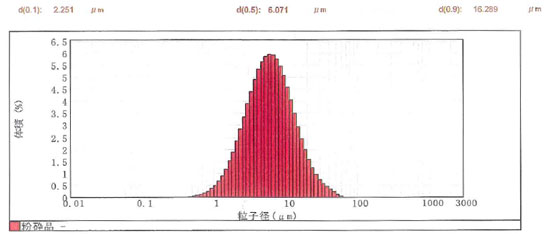
Particle size distribution of SSN-00
The table can be scrolled horizontally
| Item |
SSN-00 Analysis example |
| Purity (magnesium sulfate) |
97.8% |
| Ignition loss (moisture) |
2.2% |
| D50(average particle diameter) |
6.1μm |
| D90 |
16.3μm |
2) Ultrafine Anhydrous Magnesium Sulfate USN-00
Magnesium sulfate anhydrate is grounded to ultrafine level with a special crusher. Finer than SSN-00, realized an average particle size of 3 μm, 10 μm allpass.

Electron micrograph of USN-00 (2000 times)
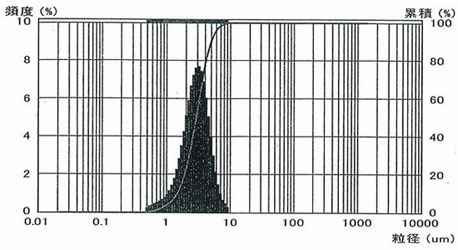
Particle size distribution of USN-00
The table can be scrolled horizontally
| Item |
USN-00 Analysis example |
SSN-00 Analysis example |
| Purity (magnesium sulfate) |
98.5% |
97.8% |
| Ignition loss (moisture) |
1.5% |
2.2% |
| D50(Average particle diameter) |
2.9μm |
6.1μm |
| D90 |
4.9μm |
16.3μm |
3) Surface Treatment Fine Particle Anhydrous Magnesium Sulfate COAT-00
When anhydrous magnesium sulfate is dissolved in water, heat of dissolution occurs. When dissolving and adjusting anhydrous magnesium sulfate in a 20% aqueous solution, the temperature rises by approximately 40 ° C (calorific value 91.2 kJ / mol).
Anhydrous magnesium sulfate is a compound also described in the additives list of the quasi-drug raw material standard 2006 and is expected to be applied as a warming material for cosmetic products such as treatments and skin care.
It is necessary to adjust the dissolution time in order to blending anhydrous magnesium in cosmetics-related products. For this reason, we are studying the dissolution time adjustment by surface treatment with a surface treatment agent.
COAT - 00 is a fine anhydrous magnesium sulfate, surface treated with a water-repellent silicone. Water-soluble magnesium sulfate floats on water by water-repellent. You stir it, and after a period of time, it dissolves gradually.

Fig. 1 Water-soluble magnesium sulfate floats on water
by water-repellent surface treatment with a water-insoluble compound Water-soluble magnesium sulfate floats on water
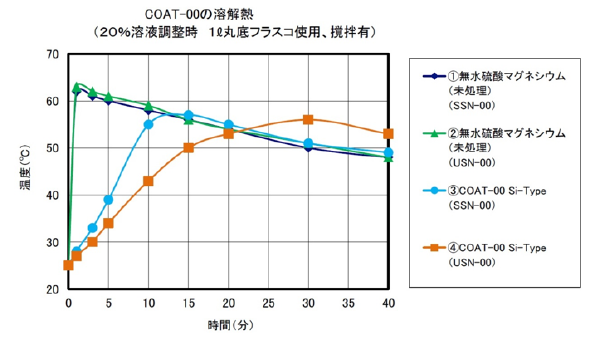
Fig. 2 Measurement result of dissolution heat
of COAT - 00 prototype (silicone surface treated product) (example)




















